Stem cells are characterized by the ability to produce copies of themselves (self-renewal) or to generate more specialized cell types (differentiation). There are 3 types of stem cells:
- Embryonic stem cells, which can be isolated and grown from early-stage embryos
- Induced pluripotent stem cells, which are similar to embryonic stem cells but reprogrammed from adult specialised cells
- Tissue or adult stem cells, which are found in our bodies all our lives and serve for tissue maintenance or repair
More information on the different types of stem cells:
Video: Diabetes and Stem Cells (source: EuroStemCell)
Pluripotent stem cells can be differentiated towards insulin-producing beta cells using knowledge from pancreas development in the embryo. This approach has been pioneered by the San Diego-based company ViaCyte. Its investigators were the first to generate human pancreatic endodermal cells (PE) from a human embryonic cell line (hu-ES) and demonstrate their in vivo differentiation to beta cell containing implants in laboratory mice, with capacity to correct diabetes. Other teams have extended this methodology to the in vitro generation of human beta cells.

Human stem cell derived pancreatic endoderm that has been manufactured in the laboratory. Cell clusters (shown in figure) contain pancreatic precursor cells that can differentiate to endocrine cells when implanted in rodents. Implants progressively develop a functional beta cell mass that controls glucose levels through insulin release.
Subcutaneous implants of embryonic stem cell-derived pancreatic endodermal cells (huES-PE) can differentiate in vivo to endocrine tissue with physiologically responsive beta cells. Human stem cell-derived grafts are conceived under encapsulated form to protect them against the immune system. Findings in animal models have led to approval for studies using the PEC-Encap™ product in man. This clinical trial undertaken in Canada and the USA has been evaluating basic safety and tolerability in patients with type 1 diabetes. Preliminary observations indicate that the product is safe and well-tolerated by patients. However, further development is needed and is underway to allow effective engraftment in patients.
In parallel to studies with the PEC-Encap™ product, ViaCyte has developed the PEC-Direct™ product candidate. It is designed to deliver the same pancreatic progenitor cells as PEC-Encap™, but in a device that allows direct vascularization. This therapy is destined to patients with type 1 diabetes that present high risks for acute complications such as hypoglycaemic coma and death. A clinical trial is currently under way in Canada and the USA to determine whether direct vascularization of implants can improve engraftment.
More information on www.viacyte.com
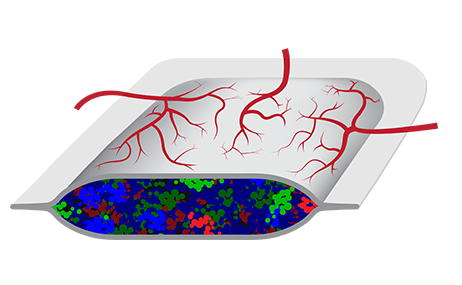
The PEC-Encap™ product is composed of stem cell-derived pancreatic tissue encapsulated in a device that prevents cell migration while allowing survival and function of the graft. Image courtesy of ViaCyte.

The PEC-Direct™ product is composed of stem cell-derived pancreatic tissue encapsulated in a device that allows direct vascularization. Image courtesy of ViaCyte.